- Adsorption is the process in which molecules or particles adhere to the surface of a solid or liquid.
- Absorption is the process in which substances are taken up and assimilated into the bulk or interior of a solid, liquid, or gas.
What is Adsorption?
Adsorption is the process where molecules or ions adhere to the surface of a solid or liquid material, forming a thin layer. This phenomenon occurs due to attractive forces, enhancing surface properties and serving various applications, such as purification and catalysis.
Example: When you sprinkle powdered sugar on a cake, the sugar particles stick to the cake's surface. This is an example of adsorption.
What does adsorptive mean?
Adsorptive refers to the capacity or characteristic of a material to attract and hold molecules on its surface through the process of adsorption, forming a thin layer of the adsorbed substance on the material's surface.
What is Absorption?
Absorption is the uptake or assimilation of substances into another substance, typically a liquid or solid. It involves the penetration and integration of molecules or particles, often leading to an increase in volume or mass.
Example
When a sponge soaks up water, the water is absorbed into the sponge's structure, filling its pores. This is an example of absorption.
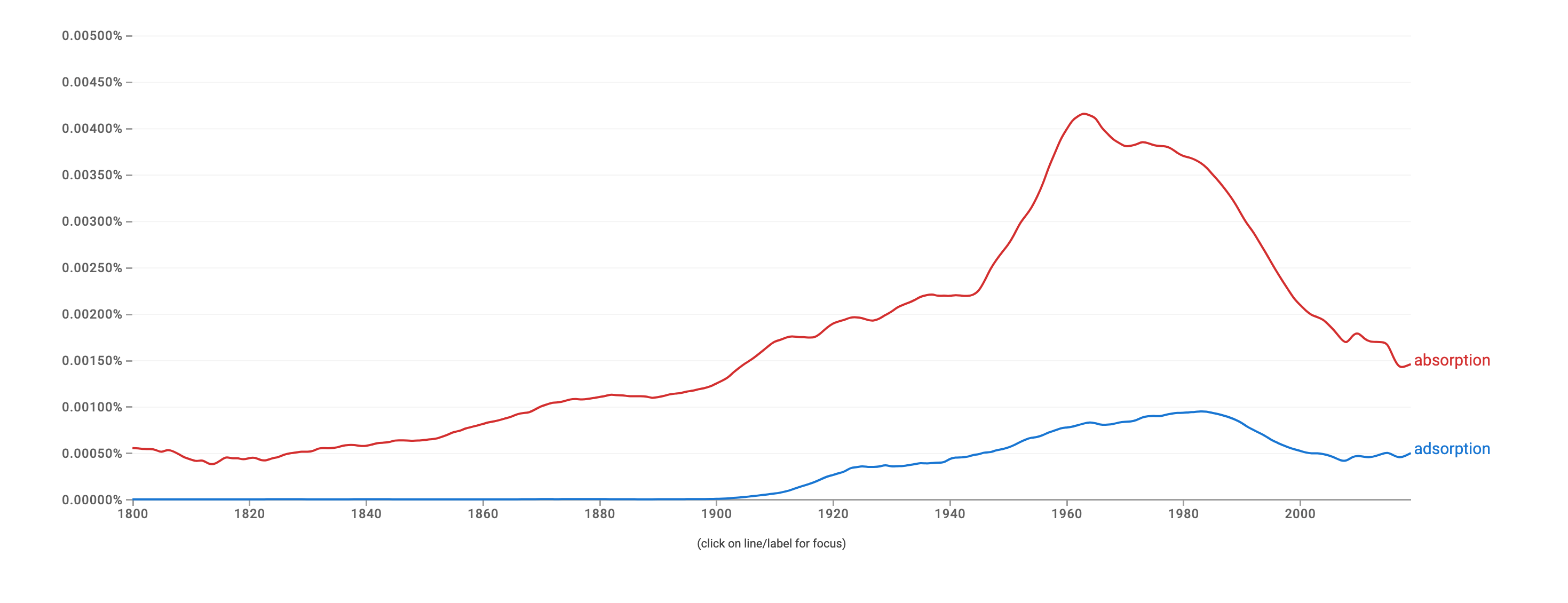
Examining the graph below, we can see that "absorption" is used more frequently than "adsorption." Both face an increase in occurrence around the 1960s and 1980s, and since then, has been on a downward trajectory.
What is the difference between adsorption and absorption?
Adsorption is the adherence of molecules to a surface, forming a thin layer without complete incorporation into the material. In contrast, absorption involves the complete integration of substances into another, causing an increase in volume or mass.
What is the difference between adsorbent and absorbent?
Adsorbents attract and hold molecules on their surface through adsorption, forming a thin layer. Absorbents, on the other hand, take in and retain substances by incorporating them throughout their structure, causing an increase in volume or mass.
Practice questions
- ____________ is the process in which molecules adhere to the surface of a solid or liquid, while ____________ involves the penetration and assimilation of substances into the bulk of a solid or liquid.
- In ____________, molecules are attracted to a surface due to Van der Waals forces or chemical bonds, whereas in ____________, molecules are taken up by the material's interior.
- When a sponge soaks up water, it is an example of ____________, whereas when a gas is captured by activated charcoal, it is an example of ____________.
- The primary driving force in ____________ is surface energy and the formation of a concentration gradient, while in ____________, it's usually chemical or physical interactions between the substance and the material.
- ____________ is commonly used in processes like water purification, where contaminants are removed by attaching to a material's surface, while ____________ is frequently employed in processes like drug delivery, where substances are taken up by a carrier material.
Answer key
- Adsorption / Absorption
- Adsorption / Absorption
- Absorption / Adsorption
- Adsorption / Absorption
- Adsorption / Absorption
Discover more about the AI English proofreader, Engram!

Reference